A condensing boiler is more efficient because it recovers some of the latent heat from the hot water vapour in the flue gas. However, no domestic condensing boiler manages to condense all the water, and how much is condensed depends on the setting of the temperature of the condenser, which is typically the ‘return temperature’ of the water in the central heating system.

Fig.1 shows how the efficiency varies with condensation temperature. (Hydrogen is slightly better at low condensing temperature, and quite a bit worse at higher temperatures.) Note that for natural gas there is no condensation at all above 55C. This is controlled by the partial pressure of water vapour which is entirely determined by the chemistry of the fuel and the quantity of inert non-condensible components in the flue gas: nitrogen, carbon dioxide and a trace of argon.
One might hope that by adding a little hydrogen to the natural gas, one might shift the dew point (the temperature at which water condenses out of the flue gas composition) to get more efficiency. Unfortunately this is a very small effect indeed, as can be seen in Fig.2

Clearly, to make a big difference we need to do something about the ~80% inert gas in the inlet air, as it is this which is diluting the water vapour and preventing it all condensing. Fig.3 shows that this is a significant effect, but condensation is still limited by all the CO2 which is produced by burning the natural gas.

There are practical limits to how much nitrogen should be removed. As progressively more nitrogen is removed, the efficiency goes up but also the adiabatic flame temperature increases. This increases the amount of poisonous nitrogen oxides produced. At 100% oxygen, this is not a problem because there is no nitrogen to be oxidised: but producing very pure oxygen is likely to be much more expensive, and the flame temperature would be so high that burners would need to be made from very expensive materials.
Oxygen-fired combustion is well understood in large industrial furnaces and the usual solution is to recirculate some of the flue gas into the flame to reduce the temperature. This may be feasible for a domestic boiler, but would increase the cost of manufacture.
We can get rid of the carbon dioxide by using a fuel which only produces water vapour: hydrogen, but we would still have the nitrogen oxides problem without flue gas (water vapour) recirculation. The efficiency gain appears to be quite significant, see Fig.4.

Fig.4 shows the very substantial increase in efficiency that occurs with oxygen enrichment of a hydrogen condensing boiler. This is such a large effect that one might expect all such boilers to employ some degree of oxygen enrichment, as (a) this would reduce the usage of the (quite expensive) hydrogen gas, and (b) hydrogen boilers need to be completely redesigned anyway, because of the different flame velocity.
Note in Fig.4 the small segment of sensible heat loss even for 100% oxygen near 100C. This is due to the excess oxygen which is not consumed by the fuel: it carries off some of the water vapour which would otherwise be condensed. All these calculations assume 15% excess air (or air mixture) to ensure complete combustion. (With a more sophisticated control system, this excess could be reduced after the boiler has started up and is running continuously, as is standard on large industrial systems.)
Figs. 5 and 6 show the same data, but as difference from the case where the fuel is burned in normal air. These figures show two kinks: one at the dew point of the reference case, the fuel burned in air, and the other at the dew point of the fuel+air mixture. These are not proportional percentage increases, these are absolute increments: the efficiency of burning natural gas goes from ~91% in air to ~94% efficient in 30% oxygen at 50C, an increase of ~3% (blue lowest line in Fig.5).


For more detail, and to see the text of the patent and links to other reports, see https://oxyboilers.co.uk .
[ Update 31 March 2024 ]
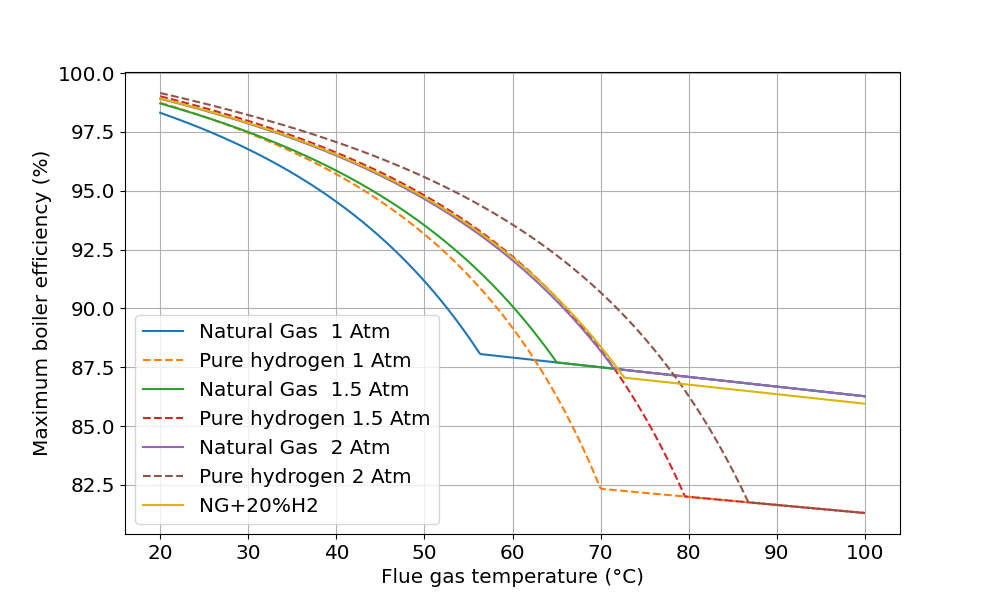
Alternatively, increase the pressure
If the condenser is at a higher pressure than atmospheric, then the partial pressure of the water vapour is too, so more of it will condense. Which makes sense intuitively as it “wants” to be in a higher density state when the pressure is higher. It doesn’t take much to shift the dew point quite usefully:
Alternative, ideal flameless boiler
The ideal solution to recovering all the energy in the water vapour would be to use a flameless cell which burned the fuel on a catalyst at the temperature of the central heating return water, or an electrochemical cell which did the same thing. The water would never be in the vapour phase, and so would not need condensing.
The code that calculates these figures is all public on GitHub. It is written in python.
The extension of this treatment to LPG boilers is left as an exercise for the student.



 Posted by philipsargent
Posted by philipsargent